Case Study: Environmental Monitoring in Pharmaceutical Industry for Meeting FDA GMP Compliance
A leading regenerative medicine company focuses on the development, manufacturing and sale of products for the wound care, and surgical and sports medicine markets. To meet FDA manufacturing regulations, the company needed a Critical Monitoring System (CMS) to collect and record environmental data to verify GMP compliance. The company wanted to have the CMS (also referred to as an EMS-Environmental Monitoring System in pharmaceutical industry) sit in parallel to the existing Building Management System (BMS), also known as a Building Automation System (BAS), and have a proven methodology to provide real-time monitoring, alerting and reporting for all sites.
CHALLENGE
In industries such as manufacturing of pharmaceutical products, facilities require both a Control (BMS) and Monitoring (EMS) solution. A BMS controls and monitors the facility’s mechanical and electrical equipment which controls the actual environment, such as the relative humidity, temperature, dew and frost point, pressure, etc. An Environmental Monitoring System in pharmaceutical industry is a GMP compliant system to verify compliance for critical environmental data regarding to both the manufacturing and storage of products.
For this company, a single facility site includes hundreds of temperature, pressure and humidity sensors wired to the BMS. In addition to those sensors, the company required additional environmental sensors, which were not currently part of the BMS. The company wanted to make the EMS flexible with the ability to add new sensors and perform calibrations without a programmer or BMS technician.
This plan triggered a larger corporate initiative to move away from disparate vendors to a singular, dedicated and compliant platform.
SOLUTION
NeoMatrix implemented Ignition by Inductive Automation and the ControlLogix Platform from Rockwell Automation utilizing PlantPAx architecture.
The team selected Ignition because its features align with 21 CFR Part 11, including the audit log, the Active Directory security support, and the Reporting Module.
NeoMatrix chose the ControlLogix platform with PlantPAx architecture as it supports the company’s methodology of utilizing a standard set of code. Using Rockwell’s PlantPAx set of tools, NeoMatrix could implement the code quicker and then configure it as needed to meet the project’s requirements. Post-deployment this use of standard code enables faster troubleshooting and makes it more easily serviceable going forward.
- FLEXIBILITY
The company wanted the ability to add I/O and calibrate I/O without any PLC or SCADA programming resources. The SCADA system was configured with the ability to securely perform those functions.
A global I/O screen features images of the I/O Rack with details for each analog point. Adding a new point can be accomplished by clicking on the point and configuring the point through a configuration popup window.
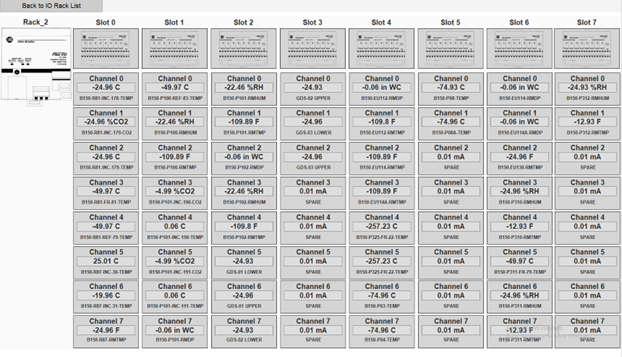
Process values can be simulated to rapidly test functionality.
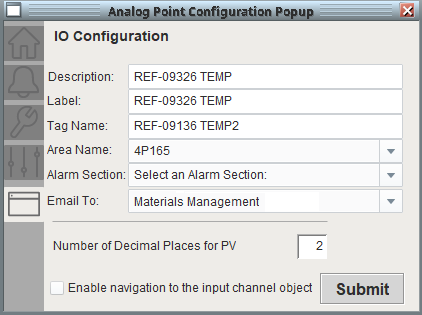
The alarm configuration dialog allows users to define the description, name and tagname properties. Specifying the area or room which the I/O point belongs to will inherit all properties of that area. That includes adding the point to any HMI graphics associated with that area, as well as what users have privileges to acknowledge any alarms associated with the I/O point.
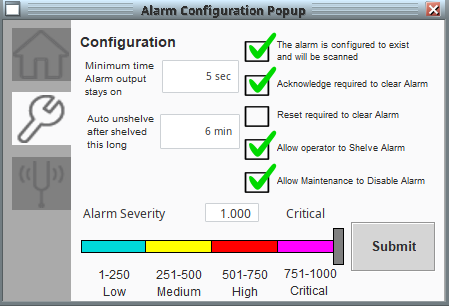
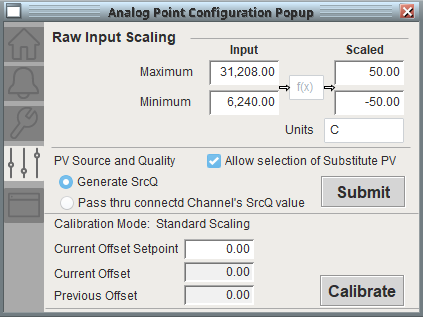
Calibration functionality was built on top of the PlantPAx objects in accordance with the company’s calibration routines. With proper security, in-house technicians can perform sensor calibrations through the HMI.
- GMP Compliance Requirements
This solution ensures that all EMS data is GMP compliant. Data records with timestamps and alarm details provide evidence that products have been manufactured and stored in ideal environmental conditions and product quality, safety, and efficacy remained intact prior to market release.
All HMI user entries are logged with username, description of activity, timestamp, and logged at time of action. The data also includes manipulation of setpoints, acknowledging or shelving of alarms, and configuration and calibration of analog points. All activities require authorized users to login via the corporate Active Directory server.
Ignition’s alarm notification pipelines are utilized to send alarm notifications via both voice and email to ensure a timely response.
Reports provide the ability to analyze audit trails and alarms, as well as point and user configurations:
- Audit trail entries are retrieved through displays information for audit trail entries. Anytime an action is taken in the CMS, it’s logged to the Audit Trail for record of actions and accessed from the Reports screen. It can be configured for a specific date range to return all audit trail events from a period.
- Alarm reports allow users to view all alarm events and acknowledgements over a range of time.
- Point configuration reports allow for the visibility of all parameters such as alarm setpoints or calibration setpoints at any specified date and time.
- The ‘Authorized User Report’ displays information for any authorized users of the system. This includes the roles the user possesses as well as the notification rosters they are a member of.
The Ignition process historian acts as a secondary data source to correlate alarm event data and troubleshooting.
All pertinent data, in accordance with regulatory requirements, resides in a SQL Server Database. The database is not accessible to users of the system and the company’s IT department maintains it with regular backups and disaster recovery plans in place.
- HIGH AVAILABILITY
NeoMatrix configured redundant Ignition servers on fault tolerant hardware. The redundant servers allow for uninterrupted data collection if a single system were to fail. The redundant servers also allow IT to perform regular system maintenance events, such as OS patches and upgrades without requiring system downtime.
RESULTS
The company will benefit from a world class CMS system that is compliant, reliable and easy to maintain. By segregating the BMS and EMS, the company can take advantage of multiple benefits, such as only validating the EMS system. Without having to validate the entire BMS system, they can avoid timely and unnecessary change control processes by the end user to adjust settings even on the smallest scale.
The independent Environmental Monitoring System will also continue to keep data records from critical locations if the BMS system experiences a software or sensor failure. For example, if a piece of product storage equipment was compromised, the EMS would verify if the environment was compromised, allowing for informed decisions to be made regarding the release or rejection of valuable product. With a platform where they have real-time monitoring and alerting of critical parameters and reporting, and data that allows them to support manufacturing and operational objectives, the company is poised for future site expansion in a cost-effective, timely and compliant manner.
WHY NEOMATRIX
The company awarded the bid to NeoMatrix given its technical competencies, which includes 20+ years of experience in the life sciences industry and extensive knowledge of utility processes such as BMS, CIP skids, steam, chilled water, clean water systems and wastewater systems. In addition to utility processes, we also have extensive expertise in the process automating cell culture, harvest, and purification processes. With this level of knowledge and experience, the client recognized NeoMatrix as the partner of choice if they wanted to shorten the time to deployment and produce the highest quality product.
NeoMatrix’s technical competencies for the project that secured the bid:
- Evaluating, designing, and implementing systems that comply to GMP/21 CFR Part 11. The systems NeoMatrix builds maintain the integrity of all GMP related data and meta data by ensuring that the information conforms to the principles of ALCOA.
- Expertise in the Rockwell Logix Platform, working extensively with the Allen Bradley PLCs ranging from PLC-5 and SLC-500 through the latest PACs in the CompactLogix and ControlLogix series processors under the GAMP (Good Automated Manufacturing Practice) 5 guidelines.
- Deep knowledge of implementing an EMS solution in conjunction with a BMS system to monitor environmental conditions that are deemed GMP-critical, including parameters such as particle counts, humidity, temperature, and pressure, and to monitor and record critical data logging of support equipment (i.e., freezers, incubators, product warehouse storage, etc.)
- As a Premier Ignition Software Partner, 100% of the company’s engineers are trained in Ignition SCADA by Inductive Automation.
NeoMatrix also develops projects in compliance with GAMP guidelines and executes based on the company’s proven process. At the beginning of the project, NeoMatrix worked with the company to refine the User Requirement Specification (URS), which included what is monitored, system requirements, regulatory requirements and a validation plan.
NeoMatrix then wrote a Functional Specification (FS) that provided details on how the design of the EMS would fulfill the requirements outlined in the URS, including the functions, operator interactions and workflows associated with the system. Included with the FS, NeoMatrix developed an alarm rationalization strategy in accordance with ISA 18.2 to reduce nuisance alarms and assure critical points are alarmed, confirming the facility is within compliance.
Understanding that a rushed specification process produces a product that falls short of expectations, NeoMatrix’s project management methodology ensures that the specification process is properly executed before any programming commences.
Help with Environmental Monitoring in Pharma
Guided by our client-first design approach and 20+ years of manufacturing technology and process experience, hundreds of leading manufacturers have trusted NeoMatrix to transform and optimize their processes.
We’ve mastered creating scalable, reliable automation and information solutions for manufacturers and industrial companies. If you’re interested in discussing how we can help improve your plant automation, regulatory compliance or enterprise Integration systems, please contact us.
You can also view our live CMS / EMS demonstration by clicking the button below.

